Stanford Researchers Claim They Found "The Holy Grail" Of Battery Life
You can't swing a dead cat these days without hitting a research team claiming to have discovered a new technology or ability that will miraculously enhance battery capabilities as soon as a few quick problems are patched up or some niggling cost factors get fixed. The majority of these announcements splash down and vanish, never to be heard from again -- but a team at Stanford is claiming to have pulled off a scientific coup that really would be a quantum leap over existing battery technology -- and they've done it, supposedly, by solving a very old problem.
Right now, the batteries we refer to as "lithium ion" use lithium in the electrolyte -- the fluid that surrounds the anode and cathode. Electrons flow from the anode into the attached device, then back into the battery via the cathode. The reason we use lithium for the electrolyte fluid but not the anode itself is simple -- lithium anodes tend to expand when they come into contact with their electrolyte solutions. As it expands, it forms tendrils of metal that cause short circuits and destroy the anode's ability to function effectively. This leads to extremely nasty problems -- problems with names like "Thermal runaway" and "Explosion.". Since turning your laptop on shouldn't require a saving throw, lithium anode batteries have had a limited market.
What Steven Chu's team claims to have discovered is a method for using hollow polystyrene nanospheres to isolate the electrolytic solution and the anode.
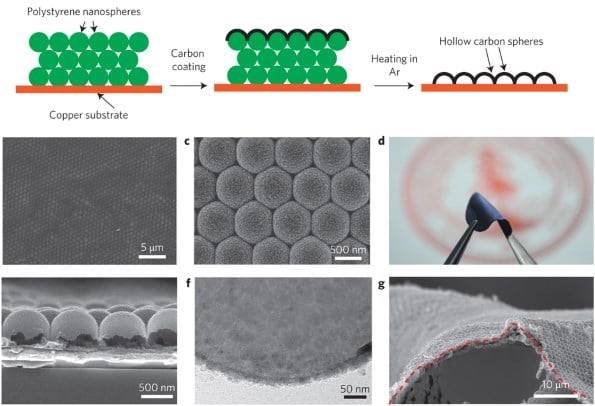
This barrier layer of carbon isolates the anode and would allow the battery to be charged and discharged repeatedly without risk of explosion. As for why this is important, check out the following graph of energy densities (originally found at Wikipedia).

This graph explains, in one swoop, why we've had such a hard time replacing oil, gas, and coal. The y-axis of the chart shows how much energy is contained within 1L of material (a measure of volume), while the x-axis is the amount of energy in 1kg (2.2lbs) of material (a measure of weight). The latter is sometimes referred to as the specific energy of a material.
Here's the simplest way to understand the chart. A kilogram of natural gas or methane contains more energy than a kilogram of diesel or gasoline (both in blue) but a liter of methane contains much less energy than either of those fuels. This makes sense -- methane is a gas, and the amount of methane you can stuff into a sports bottle at standard temperature and pressure is quite limited.
Note, meanwhile, that lithium ion batteries are sitting at the absolute bottom of the chart. They don't hold much electrical capacity per unit of volume or per unit of weight. Pure lithium, in contrast, isn't too far off diesel and gasoline.
Unfortunately, it's that same marvelous energy capacity that makes lithium so difficult to work with in consumer products -- but if Chu is correct, and we can build lithium anodes, it would open the doors for batteries 5-6x more dense than current models. Cell phones, at that point, could possibly last days on a single charge, while a car like the Tesla Model S could comfortably make a New York - LA trip without stretching for more than an overnight trickle charge.
Right now, the batteries we refer to as "lithium ion" use lithium in the electrolyte -- the fluid that surrounds the anode and cathode. Electrons flow from the anode into the attached device, then back into the battery via the cathode. The reason we use lithium for the electrolyte fluid but not the anode itself is simple -- lithium anodes tend to expand when they come into contact with their electrolyte solutions. As it expands, it forms tendrils of metal that cause short circuits and destroy the anode's ability to function effectively. This leads to extremely nasty problems -- problems with names like "Thermal runaway" and "Explosion.". Since turning your laptop on shouldn't require a saving throw, lithium anode batteries have had a limited market.
What Steven Chu's team claims to have discovered is a method for using hollow polystyrene nanospheres to isolate the electrolytic solution and the anode.

This barrier layer of carbon isolates the anode and would allow the battery to be charged and discharged repeatedly without risk of explosion. As for why this is important, check out the following graph of energy densities (originally found at Wikipedia).

This graph explains, in one swoop, why we've had such a hard time replacing oil, gas, and coal. The y-axis of the chart shows how much energy is contained within 1L of material (a measure of volume), while the x-axis is the amount of energy in 1kg (2.2lbs) of material (a measure of weight). The latter is sometimes referred to as the specific energy of a material.
Here's the simplest way to understand the chart. A kilogram of natural gas or methane contains more energy than a kilogram of diesel or gasoline (both in blue) but a liter of methane contains much less energy than either of those fuels. This makes sense -- methane is a gas, and the amount of methane you can stuff into a sports bottle at standard temperature and pressure is quite limited.
Note, meanwhile, that lithium ion batteries are sitting at the absolute bottom of the chart. They don't hold much electrical capacity per unit of volume or per unit of weight. Pure lithium, in contrast, isn't too far off diesel and gasoline.
Unfortunately, it's that same marvelous energy capacity that makes lithium so difficult to work with in consumer products -- but if Chu is correct, and we can build lithium anodes, it would open the doors for batteries 5-6x more dense than current models. Cell phones, at that point, could possibly last days on a single charge, while a car like the Tesla Model S could comfortably make a New York - LA trip without stretching for more than an overnight trickle charge.

